Webinar December 2020
A professional Webinar on the topic:
From a Conceptual “Idea” to a Marketed Product:
- Toxicology/Safety Assessment
- Pathology
- Regulatory Registration
Webinar Video
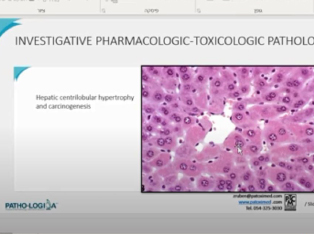
We discussed about:
- Introduction by Dr. Emmanuel Loeb, CEO, Patho-Logica, Inc.
- Definition and role of the various inter-related disciplines
- Safety assessment history (leading to the GLPs, GMPs and GCPs)
- Sources of conceptual ideas
- Discovery Phase (Research “R” leading to Development “D”)
- Pre-Clinical phase leading the IND regulatory approval
- Non-Clinical phase along Clinical Phases I, II and III and their regulatory approval
- Approval for marketing
- Post-Marketing issues related to safety