Photobiomodulation Triple Treatment in Peripheral Nerve Injury: Nerve and Muscle Response
Mira M. Mandelbaum-Livnat, PhD,1 Mara Almog, PhD,1 Moshe Nissan, PhD,1 Emmanuel Loeb, DVM,2 Yuval Shapira, MD,1 and Shimon Rochkind, MD, PhD1
Abstract
Background: Muscle preservation or decrease in muscle degeneration and progressive atrophy are major challenges in patients with severe peripheral nerve injury (PNI). Considerable interest exists in the potential therapeutic value of laser phototherapy (photobiomodulation) for restoring denervated muscle atrophy and for enhancing regeneration of severely injured peripheral nerves. As previously published, the laser phototherapy has a protective and immediate effect in PNI. Laser phototherapy in the early stages of muscle atrophy may preserve the denervated muscle by maintaining creatinine kinase (CK) activity and the amount of acet- ylcholine receptor (AChR). Objective and Methods: In the present study, the effectiveness of triple treatment laser phototherapy, namely, applied simultaneously at three areas: injured area of the peripheral nerve, cor- responding segments of the spinal cord, and corresponding denervated muscle (triple treatment), was evaluated for the treatment of incomplete PNI in rats with the ultimate goal of achieving improved limb function. Results: Forty-five days after the sciatic nerve insult, all rats regained normal walking (functional sciatic index values returned to baseline); however, the long laser irradiation (7 min) group presented the fastest recovery as opposed to short laser irradiation (3 min). A histological evaluation of the nerves revealed that long laser irradiation led to a higher amount of neuronal fibers that were larger than 4 lm (543 – 76.8, p < 0.01) than short irradiation
(283 – 35.36). A histological evaluation of muscular atrophy showed that long laser irradiation evolved with
significantly less muscle atrophy (8.06% – 1.23%, p < 0.05) than short irradiation (24.44% – 7.26%). Conclu- sions: The present study and our previous investigations showed that the laser phototherapy increases bio- chemical activity and improves morphological recovery in muscle and, thus, could have direct therapeutic applications on muscle, especially during progressive atrophy resulting from PNI.
Keywords: laser therapy, nerve regeneration, peripheral nerve injury, muscle/musculoskeletal system, nerve
Introduction
it has been estimated that the incidence of peripheral nerve injuries due to trauma is about 300,000 cases per year.1 The high prevalence of neurological lesions has prompted the medical community to search for effective so- lutions that can enhance recovery of the injured nerve and decrease the progression of muscle atrophy associated with nerve injury. Recent developments in nerve reconstructive techniques and intensive rehabilitation treatment enabled the reduction of nerve recovery time.
Among the various proposed therapeutic methods, photo- therapy received increasing attention for enhancing nerve repair. The term phototherapy refers to the use of light for producing a therapeutic effect on living tissues. An extensive review of the literature2 showed that more than 80% of the experimental studies carried out on the use of laser photo- therapy for promoting peripheral nerve repair led to a positive outcome on post-traumatic/postoperative nerve recovery.
The restoration of the injured peripheral nerve prevents the progression of the muscle atrophy process and allows functional recovery. However, restoring functions in the case of long-term peripheral nerve injury (PNI) is still dif- ficult, since progressive muscle atrophy sets in shortly after nerve injury.3–7 For this reason, therapeutic solutions that can lessen muscle degeneration during the period of nerve recovery can increase the probability of early recuperation of functional motor activity. In the past decade, interest in the therapeutic effect of laser phototherapy on muscle has risen sharply.
The present study applied an experimental peripheral nerve crush model to test the impact of triple treatment laser photo- therapy on atrophic gastrocnemius muscle in case of incom- plete PNI. The triple treatment includes irradiation on three areas: injured peripheral nerve, corresponding segments of spinal cord, and corresponding denervated muscle (gastrocne- mius). This was performed to evaluate the added value of irradiating the corresponding muscle on the injured nerve recovery. The impact of laser phototherapy was evaluated by using two irradiation periods, 3 min irradiation and 7 min irra- diation, to evaluate the effect of the amount of energy delivered.
Materials and Methods
A blind, randomized controlled study was performed to evaluate the efficacy of triple treatment laser phototherapy as a treatment for: (1) incomplete PNI—crush injury model, and (2) muscle preservation after crush PNI and to evaluate the effect of short and long laser irradiation. All animal experiments were approved by the Institutional Animal Care and Usage Committee and adhered strictly to the Animal Care guidelines.
Thirty female Wistar rats, weighing 200–250 g each, were brought to the vivarium 2 weeks before the surgery and housed two per cage with a 12-h light/dark cycle, with free access to food and water. The animals were marked, and each animal was ascribed to a test group by an independent researcher. The study was conducted by using an experimental model for producing an incomplete PNI that has been described.8,9
General anesthesia was induced with an intraperitoneal injection of xylazine (15 mg) and ketamine (50 mg). All surgical procedures were performed by using aseptic sur- gical techniques and under a high magnification microscope. Depilation of the surgical site was accomplished with an electric animal clipper. The area was vacuumed to remove hair clippings and debris, and it was then rinsed with alcohol (Alcohol Chlorexdine 0.5% w/v Chlorexdidine Gluconate in 70% v/v Isopropanol).
The left sciatic nerve was exposed and separated from biceps femoris and semimembranosus muscles, beginning from the area of branches to the glutei and hamstring muscles and distally to the trifurcation into peroneal, tibial, and sural nerves. The incomplete sciatic nerve injury was induced by the standard method of 30 sec crush by using a standard hemostat. The muscular, subcutaneous, and skin layers were closed by using silk 3–0 sutures. Rats were divided into three experimental groups: 3 min laser irradia-
tion (n = 10); 7 min laser irradiation (n = 10); and control group with no laser irradiation (n = 10).
Laser irradiation
Rats were induced under general anesthesia with an in- traperitoneal injection of xylazine (15 mg) and ketamine (50 mg). The control group was given anesthesia for 14 consecutive days with no further intervention, whereas the other groups were followed by 780 nm laser irradiation for 14 consecutive days, in accordance with their affiliation: (1) 3 min laser irradiation and (2) 7 min laser irradiation to each of the irradiated areas.
During laser irradiation, the rats were placed on the ab- domen and the laser was placed above the three irradiated areas: (1) injured area of the peripheral nerve, (2) corre- sponding segments of the spinal cord, and (3) corresponding gastrocnemius muscle. The laser was calibrated before ir- radiation; then, it was placed 11 cm above the skin, and its power was set at 250 mW with a spot size of 5 · 6 mm (1.061 W/cm2). In 3 min per spot group, the energy was P · T = 250 mW · (3 · 60) sec = 45 J, and the fluence was
45 J/0.2356 cm2 = 191 J/cm2. In 7 min per spot group, the energy was P · T = 250 mW · (7 · 60) sec = 105 J, and the fluence was 105 J/0.2356 cm2 = 446 J/cm2.
Functional evaluation
Functional sciatic index (FSI) evaluates the functionality of the operated limb compared with the intact limb. After dipping the rat’s hindlimbs in black nontoxic ink, it is re- leased to walk along a 40 cm-long, 10 cm-wide tract, with sealed sides and top, ending in a dark box. The ink on the ambulating rat’s hindlimbs leaves tracks on an underlying paper. These imprints have basic characteristics, such as maximal distance between anterior and posterior footprint margins (PL), distance between fingerprints 1–5 (TS) and 2– 4 (ITS). The formula used for measuring FSI is:
FSI ¼ – 38:3 · (EPL – NPL)=NPL þ 109:5
- (ETS – NTS)=NTS þ 13:3
- (EITS – NITS)=NITS – 8:8:
The letter N or E before the variables denotes Normal or Experimental limbs, respectively. An FSI value of 0 indicates a good functional recovery of the operated limb compared with the intact limb. The closer the number is to 100, the more complete is the denervation of the limb. The FSI test was conducted preoperatively (baseline) and at 7 (during laser irradiation period), 14 (at the end of laser irradiation period), and 45 days postoperatively (at the end of the study).
Histology evaluation
The rats were sacrificed by lethal doses of CO2 45 days after the surgical procedure. Thereafter, sciatic nerves and muscles were collected from all tested rats. Sciatic nerve samples were taken both proximally and distally to the in- jury site and fixed in 10% neutral buffered formalin (*4% formaldehyde solution).
The sciatic nerve was cut into three different cross- sections: proximal, mid, and distal. The muscles were kept in fixative for a routine H&E staining. Tissues were processed routinely, embedded in paraffin, and sectioned on a Lika microtome at 7 lm thickness. Deparaffinization and rehy- dration of sections were performed according to classical procedures. Slides were placed in xylene for 10 min (three times) followed by a serial 5 min wash in ethanol (100%, 95%, 70%), and they were finally rinsed in PBS (three times). Sections were placed in a Coplin jar with dilute antigen retrieval solution (10 mM citrate acid, pH 6), and they were heated in a microwave to 95°C–100°C for 10 min.
After rinsing in PBS (three times), the slides were blocked with 20% normal horse serum in PBS for 1 h at 37°C and they were incubated with first antibody [CHat or NeuroFilament (NF)] in 2% normal horse serum in PBS ON at RT. CHat stains only motor neuron fibers, whereas NF stains all nerve fibers. The next day, after three washes with PBS, slides were incubated with biotinylated (AP)- conjugated secondary antibody for 1 h at RT. The slides were washed with PBS (three times) and were incubated for an additional hour with cy3-conjugated streptavidin. After three washes with PBS, the slides were cover-slipped with aqua PolyMount.
The slides were visualized under a fluorescent micro- scope, and photographs were taken with a high-resolution camera (E-600; Nikon, Kawasaki, Japan) that was con- nected to a computer. For each sample, two photos were taken: only CHat staining and a merged picture with both CHat and NF. The samples were evaluated by using a fluorescence microscope (20 · increase), counting both the number of nerve fibers and the sizes of the fibers (under 4 lm or above it).
A blinded digital morphometric analysis was performed by using Image-Pro Plus 4.1 The area of the Chat-positive reaction (functional motor neuron), versus area of total neurons (NF) is measured to count the number of neurofi- laments in each sample, both proximal and distal to the crush injury area, in relation to the fiber’s size: (1) NF <4— the amount of all fibers that are smaller than 4 lm; (2) CHat
<4—the amount of motor fibers that are smaller than 4 lm;
(3) NF >4—the amount of all fibers that are larger than 4 lm; and (4) CHat >4—the amount of motor fibers that are larger than 4 lm. Fibers that are larger than 4 lm are con- sidered better, therefore a higher amount of fibers, especially motor fibers that are larger than 4 lm, points toward better regeneration.10
A histopathological evaluation was carried out to assess the regeneration of the neuronal fibers after a crush injury and to assess the efficacy of various treatments.
The condition of the gastrocnemius muscles was assessed histologically by using a fluorescence microscope. The gastrocnemius muscles of 30 rats were fixed in 10% neutral buffered formalin (*4% formaldehyde solution). From each muscle, a cross-section and a longitudinal cut were made. Tissues were trimmed, embedded in paraffin, sec- tioned at *2–3 lm thickness, and stained with HE stain.
Thereafter, they were evaluated for the grade of atrophy by using the percentage of loss of myofibers per 10 · mag- nification: 0 = normal muscle (compared with the normal, untreated nerve in the right leg); 1 = mild atrophy with loss of up to 10% myofibers; 2 = moderate atrophy with loss of up to 50% myofibers; and 3 = severe atrophy with loss of more than 50% myofibers. To have a more accurate analysis of results, a further evaluation of the absolute number of atrophy percentage was performed.
Statistical analysis
All statistical analysis and calculations were performed by MatLab software (Ver. 2008b; The MathWorks, Inc.). The data analysis of the nerve samples (histology) was carried out on a total area of 146,000 lm2 and sampled for cells that were colored with NF (color all nerve fibers) and Chat (color only motor nerves). Due to the small group size, a nonparametric analysis was performed: Wilcoxon signed- rank test (p) for related samples. Statistical significance was calculated for each parameter [type of nerve and location on the nerve (proximal versus distal)], in addition to proximal/ distal ratio between all groups.
The muscle atrophy data analysis was carried out on a total of 60 rats, since another control was added—the other limb with no insult. The state of the muscle was noted as well on the following grade scale: (0) normal muscle (com- pared with the normal, untreated nerve in the right leg); (1) mild atrophy with loss of up to 10% myofibers per 10 · magnification; (2) moderate atrophy with loss of up to 50% myofibers per 10 · magnification; and (3) severe atrophy with loss of more than 50% myofibers per 10 · magnification. Statistical analysis was calculated on the raw data of the loss percent, and parametric analysis was applied: The data were average with standard deviation. Statistical significance was calculated by using Student’s t distribution (t-test).
Results
Peripheral nerve regeneration analysis
A functional evaluation was performed before insult, 7 days postsurgery (the middle of irradiation period), 14 days postsurgery (the end of irradiation period), and 45 days postsurgery (end of study). Forty-five days after the injury, all rats returned to normal walking according to FSI; however, the pattern of recovery was different among the groups. The group that underwent 7 min irradiation was shown to evolve with faster recovery in comparison to 3 min irradiation and control (no irradiation). Interestingly, the 3 min irradiation evolved with the slowest recovery pattern (Fig. 1). Due to a major problem of autotomy (self-eating of fingers), it was not possible to perform a statistical analysis; however, the response patterns were clearly seen.
A histological and morphometric analysis was per- formed to evaluate the effect of laser irradiation on the amount of neuronal fibers, including motor fibers, and the quality of the fibers. The division according to quality was based on fibers greater than 4 lm and smaller than 4 lm.
A comparison of the amount of neuronal fibers between short (3 min) and long laser irradiation (7 min) revealed a significant difference ( p < 0.01); whereas irradiation for 7 min evolved with a higher amount of all fibers larger than 4 lm at the distal position, 543 – 76.8 as opposed to 283 – 35.36. However, a comparison to the control exhibited significant differences compared with the short irradiation, where short irradiation evolved with less neuronal fibers larger than 4 lm at the distal position, 283 – 35.36 as op- posed to 555 – 54.16. A comparison of motor fibers evolved with significant differences (p < 0.05) at the distal position in fibers larger than 4 lm, 96 – 44.88 as opposed to 186 – 53.12 (Figs. 2 and 3).
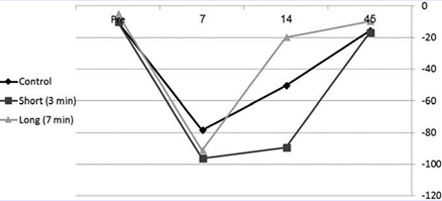

FIG. 1. FSI—a comparison be- tween short (3 min) and long laser ir- radiation (7 min) to control (crush injury with no further treatment). Rhombus—control, square—short laser irradiation, triangle—long laser irradiation. Pre-evaluation of FSI before sciatic nerve injury and 7, 14, and 45—number of days after sciatic nerve injury. FSI, functional sciatic index.
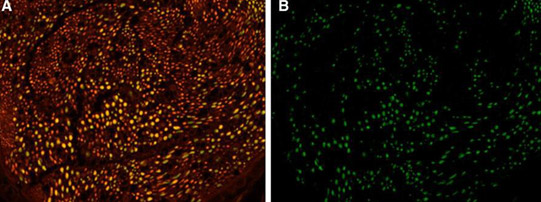

FIG. 2. Cross-section of sciatic nerves stained with NF (stains all NF) and CHATt (stains motor fibers). 4 · magnification
- NF merged with chat. Yellow—cross-linking of NF and Chat—motor axons. Red—other NFs. (B) Chat staining. Green—motor axons. NF,
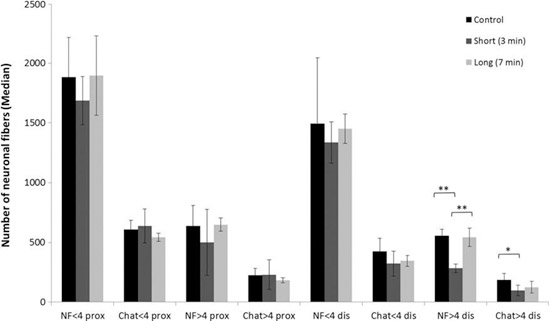

FIG. 3. A comparison of control, short, and long laser irradiation in relation to the number of nuronal fibers in both the proximal and distal areas. Results are presented in median – mad. *Significant difference of p < 0.05 and **significant difference of p < 0.01. Black—control, dark gray—short laser irradiation, and bright gray—long laser irradiation. NF— staining of all nuronal fibers. Chat—staining of motor fibers. NF <4 prox—neuronal fibers smaller than 4 lm at the proximal area, Chat <4 prox—motor fibers smaller than 4 lm at the proximal area, NF >4 prox—neuronal fibers larger than 4 lm at the proximal area, Chat >4 prox—motor fibers larger than 4 lm at the proximal area, NF <4 dis—neuronal fibers smaller than 4 lm at the distal area, Chat <4 dis—motor fibers smaller than 4 lm at the distal area, NF >4 dis—neuronal fibers larger than 4 lm at the distal area, and Chat >4 dis—motor fibers larger than 4 lm at the distal area.
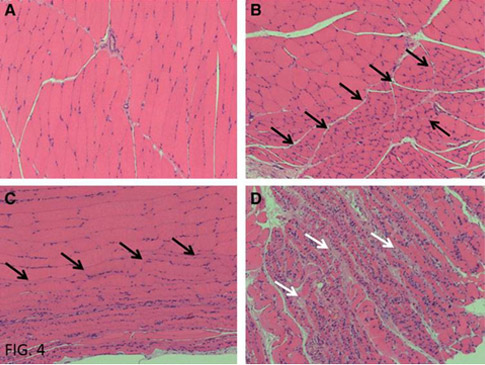

FIG. 4. Levels of muscular atrophy. The gastrocnemius muscles were stained by us- ing an HE stain and evalu- ated for the grade of atrophy per 10 · magnification. (A) Grade 0—monotonic myofi- ber volume. (B) Grade 1—A focal area of atrophy (black arrows) with hypercellular condition. Note the small volume of the myofibers. Purple—satellite cells. (C) Grade 2—a large area of at- rophy (black arrows) with hypercellular condition. Note the small volume of the myofibers. (D) Grade 3—a diffuse area of atrophy with hypercellular condition. Note the small volume of myofi- bers and the increase of col- lagen fibers (fibrosis)—white arrows and the large amount of satellite cells.
Muscular atrophy analysis
The muscle atrophy grade of the healthy limb was 0 in all rats, whereas the muscle atrophy grade of the control, non- irradiated insulted limb ranged between 0 and 35. In the group irradiated for 3 min, it ranged between 10 and 65; whereas in the group irradiated for 7 min, it ranged between 0 and 10. A statistical evaluation of muscular atrophy (Figs. 4 and 5) revealed that laser irradiation for 7 min evolved with significantly less muscle atrophy (8.06% – 1.23%, p < 0.05), as opposed to 3 min of irradiation (24.44% – 7.26%), but with no significant difference in the control group.
Discussion
PNI, complete or incomplete, is common. The recovery process for a peripheral nerve-injured patient, either with or without surgical intervention, is lengthy in duration (ranges from 6 to 24 months) and often incomplete. The current treatments, such as physiotherapy, occupational therapy, and electrical stimulation, are insufficient, especially in cases of severe nerve injury. In the majority of cases, patients remain with a loss of sensory and motor functions, which lead to severe occupational and social consequences.
The beneficial effect of laser phototherapy on injured peripheral nerves was already published extensively.2,11,12 Moreover, 780 nm laser phototherapy was found to stimu- late migration and fiber sprouting of neuronal cell aggre- gates, enhancing the development of large-sized neurons with a dense, branched interconnected network of neuronal fibers.13 However, most of the studies focus on treatment of the site of the nerve injury only; whereas the main goal of restorative medicine is muscle preservation and the preven- tion of often debilitating joint contractures.
When muscles are denervated, they deteriorate progres- sively. Prolonged denervation causes multiple functional and morphological changes in skeletal muscle due to the absence of motor and trophic regulatory control by the nerve. The most prominent features of denervated skeletal muscles are the rapid atrophy of muscle fibers and a de- crease in the number of both myonuclei and satellite cells.4,6,14 One of the main explanations for the incomplete restoration of very long-term denervated muscle is the failure of regenerating nerves to reach all of the atrophic muscle fibers and to establish mature muscle–nerve con- tacts.15 If not reinnervated, the regenerating myofibers un- dergo atrophy and degeneration. To lessen or temporarily prevent this process, laser phototherapy may be an effective tool that preserves denervated muscle until nerve sprouting into the muscle occurs.
Our previous work has indicated that the use of low- power laser irradiation on the injury site and/or on the spinal cord segment related to the injured nerve enhances the re- habilitation process of the injured limb. Further, it was found that irradiation of the denervated muscle assists in prevention of muscle atrophy.16–18
In the current study, the effect of triple treatment laser phototherapy was evaluated, namely, irradiation of three ar- eas: injured peripheral nerve, corresponding segment of spi- nal cord, and corresponding muscle. This was evaluated in two lengths of irradiation influencing the amount of energy delivered: 3 and 7 min irradiation. We found that the amount of energy delivered to the rat affects total nerve regeneration. The triple treatment irradiation of 780 nm for 7 min was more effective in promoting total nerve regeneration than 3 min irradiation. Further, laser phototherapy significantly de- creased the atrophy of corresponding muscles.
Triple treat- ment irradiation was found to preserve muscle from denervation. Irradiation of 7 min was shown to better preserve from muscle denervation than 3 min irradiation.
These results suggest that photobiomodulation treatment can accelerate muscle restoration during the postinjury pe- riod, and it could have direct therapeutic applications for the preservation of denervated muscle after PNI.
Further studies are underway, and they will include function assessment and electrophysiological assessment. Promising results could lead to the eventual application of photobiomodulation triple treatment in the clinical practice for achieving improved limb function in patients with in- complete PNI.
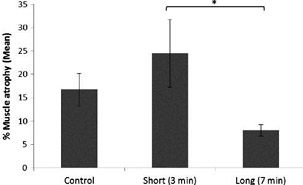

FIG. 5. Muscle atrophy—a comparison of all groups. Results are presented as mean – SE. *Significant difference of p < 0.05. Control—crush injury with no further treatment, Short—short laser irradiation (3 min), and Long—long laser irradiation (7 min).
Possible mechanism
We have previously suggested that the function of dener- vated muscles can be partially preserved by temporary pre- vention of denervation—induced biochemical changes.17,18 It was found that He-Ne laser treatment of lesioned muscles could increase mitochondrial activity in muscular fibers, activate fibroblasts19 and macrophages, and stimulate an- giogenesis.15 In addition, irradiation of muscle cell cultures (632.8 nm, 3 J/cm2, 20 mW) led to a rise in the levels of the neurotropic nerve growth factor, secreted by skeletal mus- cles that influence the survival and regeneration of sympa- thetic neurons in the peripheral nervous system.20 Other neurotropic growth factors have been reported to be biosti- mulated by laser therapy, such as GAP-43.21
It was demonstrated that laser treatment promotes an in- crease in Collagen IV immunolabeling in skeletal muscle after cryoinjury22 and decreases IL-1B expression during the skel- etal muscle repair after acute injury.23 Further, laser irradiation on intact muscle, muscle cells,16 and denervated muscle18 could have a therapeutic effect that induces biochemical changes, which might be a trophic signal for increased activity of creatinine kinase (CK), thus preserving a reservoir of high-energy phosphate that is available for quick resynthesis of ATP and that would enable the survival of AChR.
The data collected from different experimental studies support our results and help in understanding the mechanism of influence of low-power laser irradiation (visible and near- infrared wavelengths) and muscle tissue. It has been demon- strated that reactive oxygen species formation, oxidative damage markers, and augmented collagen synthesis are eli- cited by traumatic muscular injury, effects that are signifi- cantly decreased by laser treatment.24 An evaluation of mitochondrial respiratory chain complexes and succinate de- hydrogenase activities after traumatic muscular injury showed that the laser treatment may induce an increase in ATP syn- thesis, and that this may accelerate the muscle-healing pro- cess25 and delay fusion of cultured myoblasts.26 The increase of muscle fiber area and mitochondrial density after laser treatment was reported after muscle toxic injury.27,28
The process of regeneration in denervated muscles was markedly enhanced in muscle that was irradiated by laser before injury, probably by activation (stimulation of pro- liferation and/or differentiation) of cells in the muscles that are ‘‘recruited’’ and participate in the process of regenera- tion.18,29 In the model of prolonged muscle ischemia, laser treatment decreased post-traumatic changes in creatine ki- nase and lactate dehydrogenase.30 A positive effect on muscle metabolism was found after cryolesion injury, whereas cyclo-oxyge 2 (COX-2) immunoexpression was lower in the laser-treated group. COX-2 is a key enzyme in the conversion of arachidonic acid to prostanoids.31 The expression of COX-2 is relevant to many pathological pro- cesses, including inflammation and tissue repair.
Recently, we found18 that laser irradiation on the dener- vated muscle has a significant protective effect at two time periods: during the first period of 21 days for AChR and at 30 days for CK activity. We found that in the early stages of muscle degeneration, laser treatment may temporarily pre- serve AChR and CK in the denervated muscle close to its initial level before injury, and it may partially maintain CK activity and the amount of AChR in the denervated muscle during the consecutive stages of muscle degeneration. This laser effect confirms our previous results on muscle cells and intact muscles.16
At the cellular level, we also found increased DNA syn- thesis and CK activity in young and mature skeletal muscle. The induced biochemical changes may be attributed to trophic signals for increased activity of CK, thus preserving a reservoir of high-energy phosphate that is available for quick resynthesis of ATP. These findings are supported by early results by Bo- lognani and Volpi32 and Passarella et al.33 and recently by Ferraresi et al.,34 who showed that laser irradiation increased ATP production in the mitochondria. The biochemical cascade that occurs in the affected muscle eventually enhances mor- phological muscle recovery that is shown in the present study. The current study and our previous publications16,18 as well as other reports 22–24,35 suggest that laser phototherapy may en- hance biochemical activity and morphological recovery of the muscle to overcome stress conditions.
Conclusions
The present study shows that laser phototherapy improves morphological recovery in muscle and, thus, could have direct therapeutic applications on muscle, especially during progressive atrophy resulting from PNI.
Acknowledgments
This work was supported by the Israeli Ministry of De- fense, Grant No. 2500. The authors thank Mr. Igal Koifman for his guidance on laser devices.
Author Disclosure Statement
No competing financial interests exist.
References
- Ciardelli G, Chiono V. Materials for peripheral nerve re- generation. Macromol Biosci 2006;6:13–26.
- Gigo-Benato D, Geuna S, Rochkind S. Phototherapy for enhancing peripheral nerve repair: a review of the litera- ture. Muscle Nerve 2005;31:694–701.
- Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int 2014;2014:698256.
- Borisov AB, Carlson BM. Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec 2000;258:305–318.
- Higashino K, Matsuura T, Suganuma K, Yukata K, Nish- isho T, Yasui N. Early changes in muscle atrophy and muscle fiber type conversion after spinal cord transection and peripheral nerve transection in rats. J Neuroeng Rehabil 2013;10:46.
- Schmalbruch H, Lewis DM. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve 2000;23:617–626.
- Selcen D. Muscle diseases. In: Goldman Cecil Medicine. 25th ed. L Goldman, AI Schafer (eds.). Philadelphia, PA: Elsevier Saunders, 2016; Chap. 421; 2537–2547.
- Rochkind S, Nissan M, Barr-Nea L, Razon N, Schwartz M, Bartal A. Response of peripheral nerve to He-Ne laser: experimental studies. Lasers Surg Med 1987;7: 441–443.
- Rochkind S, Barr-Nea L, Razon N, Bartal A, Schwartz
- Stimulatory effect of He-Ne low dose laser on in- jured sciatic nerves in rats. Neurosurgery 1987;20:843– 884.
- Shamir MH, Rochkind S, Sandbank J, Alon M. Double- blind randomized study evaluating regeneration of the rat transected sciatic nerve after suturing and postoperative low-power laser treatment. J Reconstr Microsurg 2001; 17:133–138.
- Wang C-Z, Chen Y-J, Wang Y-H, et al. Low-level laser irradiation improves functional recovery and nerve regen- eration in sciatic nerve crush rat injury model. PLoS One 2014;9:e103348.
- Rochkind S. Phototherapy in peripheral nerve regeneration: from basic science to clinical study. Neurosurg Focus 2009;26:E8.
- Rochkind S, El-Ani D, Nevo Z, Shahar A. Increase of neuronal sprouting and migration using 780 nm laser pho- totherapy as procedure for cell therapy. Lasers Surg Med 2009;41:277–281.
- Bongers KS, Fox DK, Ebert SM, et al. Skeletal muscle denervation causes skeletal muscle atrophy through a pathway that involves both Gadd45a and HDAC4. Am J Physiol Endocrinol Metab 2013;305:E907–E915.
- Iyomasa DM, Garavelo I, Iyomasa MM, Watanabe IS, Issa JP. Ultrastructural analysis of the low level laser therapy effects on the lesioned anterior tibial muscle in the gerbil. Micron 2009;40:413–418.
- Rochkind S, Geuna S, Shainberg A. Phototherapy and nerve injury: focus on muscle response. Inter Rew Neuro- biol 2013;109:99–109.
- Rochkind S, Geuna S, Shainberg A. Chapter 25: Photo- therapy in peripheral nerve injury: effects on muscle pres- ervation and nerve regeneration. Int Rev Neurobiol 2009;87:445–464.
- Rochkind S, Shainberg A. Protective effect of laser pho- totherapy on acetylcholine receptors and creatine kinase activity in denervated muscle. Photomed Laser Surg 2013;31:499–504.
- Ihsan FR. Low-level laser therapy accelerates collateral circulation and enhances microcirculation. Photomed Laser Surg 2005;23:289–294.
- Schwartz F, Brodie C, Appel E, Kazimirsky G, Shainberg
- Effect of helium/neon laser irradiation on nerve growth factor synthesis and secretion in skeletal muscle cultures. J Photochem Photobiol B 2002;66:195–200.
- Shin DH, Lee E, Hyun JK, et al. Growth-associated protein-43 is elevated in the injured rat sciatic nerve after low power laser irradiation. Neurosci Lett 2003;344: 71–74.
- Baptista J, Martins MD, Pavesi VC, et al. Influence of laser photobiomodulation on collagen IV during skeletal muscle tissue remodeling after injury in rats. Photomed Laser Surg 2011;29:11–17.
- Fernandes KP, Alves AN, Nunes FD, et al. Effect of pho- tobiomodulation on expression of IL-1b in skeletal muscle following acute injury. Lasers Med Sci 2013;28:1043–1046.
- Silveira PC, da Silva LA, Pinho CA, et al. Effects of low-level laser therapy (GaAs) in an animal model of muscular damage induced by trauma. Lasers Med Sci 2013;28:431–436.
- Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R. Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J Photochem Photobiol B 2009;95:89–92.
- Wollman Y, Rochkind S. Muscle fiber formation in vitro is delayed by low power laser irradiation. J Photochem Pho- tobiol B 1993;17:287–290.
- Amaral AC, Parizotto NA, Salvini TF. Dose-dependency of low-energy HeNe laser effect in regeneration of skeletal muscle in mice. Lasers Med Sci 2001;16:44–51.
- Assis L, Yamashita F, Magri AMP, Fernandes KR, Ya- mauchi L, Renno ACM. Effect of low-level laser therapy (808 nm) on skeletal muscle after endurance exercise training in rats. Braz J Phys Ther 2015;19:457–465.
- Bibikova A, Oron U. Regeneration in denervated toad (Bufo viridis) gastrocnemius muscle and the promotion of the process by low energy laser irradiation. Anat Rec 1995;241:123–128.
- Lakyova´ L, Toporcer T, Tomevckova´ V, Sabo J, Radonˇak J. Low-level laser therapy for protection against skeletal muscle damage after ischemia-reperfusion injury in rat hindlimbs. Lasers Surg Med 2010;42:665–672.
- Renno´ AC, Toma RL, Feitosa SM, et Comparative effects of low-intensity pulsed ultrasound and low-level laser therapy on injured skeletal muscle. Photomed Laser Surg 2011;29:5–10.
- Bolognani L, Volpi N. Low power laser enzymology: re- activation of myosin ATPase by GaAs and HeNe lasers. In: Basic and Applied Research in Photobiology and Photo- medicine. S Passarella, E Quadliariello (eds.). Bari, PA: Uni- versity of Bari, 1991; pp. 21–42.
- Passarella S, Casamassima E, Molinari S, et al. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett 1984;175:95–99.
- Ferraresi C, Kaippert B, Avci P, et al. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak re- sponse at 3–6 h. Photochem Photobiol 2015:91:411–416.
- Belchior AC, dos Reis FA, Nicolau RA, Silva IS, Perreira DM, de Carvalho Pde T. Influence of laser (660 nm) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med Sci 2009;24:893–899.
Address correspondence to:
Shimon Rochkind Division of Peripheral Nerve Reconstruction
Department of Neurosurgery Tel Aviv Sourasky Medical Center
Tel Aviv University 6 Weizmann Street
Tel Aviv 64239
Israel E-mail: rochkind@zahav.net.il
Received: February 2, 2016.
Accepted after revision: August 11, 2016.
Published online: December 8, 2016.